
Training course in data analysis for genomic surveillance of African malaria vectors
Workshop 7 - Gene flow and the spread of insecticide resistance
Module 1 - Organophosphate resistance markers (combining SNP and CNV data)#
Theme: Analysis
The acetylcholinesterase enzyme produced by the Ace1 gene is the target for organophosphate insecticides. Both target-site mutations and gene copy number amplifications have been implicated in insecticide resistance phenotypes.
Overexpression of the Gste2 gene has also been associated with resistance to some organophosphates, and SNPs in the same gene have been linked to pyrethroid and DDT resistance phenotypes.
In this module we’re going to investigate known organophosphate insecticide resistance markers, learning how to combine SNPs and CNVs, using data from Ag3.0.
Learning Objectives#
Understand mechanisms and markers of organophosphate resistance.
Revisit amino acid frequency and CNV gene frequency analyses.
Learn how to combine and interpret SNP and CNV frequency data.
Lecture#
English#
Français#
Please note that the code in the cells below might differ from that shown in the video. This can happen because Python packages and their dependencies change due to updates, necessitating tweaks to the code.
Setup#
First, let’s begin by installing and importing some Python packages, and configuring access to Anopheles genomic data from the MalariaGEN Ag3.0 data resource.
%pip install -q --no-warn-conflicts malariagen_data
import malariagen_data
import warnings
warnings.simplefilter(action='ignore', category=Warning)
import pandas as pd
import plotly.io as pio
pio.renderers.default = "notebook+colab"
Note that authentication is required to access data through the package, please follow the instructions here.
ag3 = malariagen_data.Ag3()
ag3
| MalariaGEN Ag3 API client | |
|---|---|
| Please note that data are subject to terms of use, for more information see the MalariaGEN website or contact support@malariagen.net. See also the Ag3 API docs. | |
| Storage URL | gs://vo_agam_release_master_us_central1 |
| Data releases available | 3.0, 3.1, 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 3.10, 3.11, 3.12, 3.13, 3.14 |
| Results cache | None |
| Cohorts analysis | 20250502 |
| AIM analysis | 20220528 |
| Site filters analysis | dt_20200416 |
| Software version | malariagen_data 15.2.0 |
| Client location | Iowa, United States (Google Cloud us-central1) |
Introduction#
The Ace1 gene#
Acetylcholinesterase, also known as AChE, is an essential carboxylesterase enzyme in the nervous system of animals. AChE breaks down the neurotransmitter acetylcholine.
In anophelines, the AChE protein is produced by the Ace1 gene.
In the diagram below we see the neurotransmitter acetylcholine (ACh) being released from the pre-synaptic membrane of one neuron in the nervous system, and binding to receptors on the post-synaptic membrane of another neuron. This enables a nerve impulse to travel between the neurons. AChE (the red “Pac Man”) breaks ACh down into acetate and choline, terminating the nerve impulse.

Figure reference: Muramatsu I, Masuoka T, Uwada J, et al. A New Aspect of Cholinergic Transmission in the Central Nervous System. 2018 Apr 4. In: Akaike A, Shimohama S, Misu Y, editors. Nicotinic Acetylcholine Receptor Signaling in Neuroprotection [Internet]. Singapore: Springer; 2018. http://creativecommons.org/licenses/by/4.0/
Resistance to arganophosphate and carbamate insecticides#
Organophosphate (OP) and carbamate insecticides block the action of AChE via competitive binding. This results in nerve impulses not being terminated, and leads to insect death.
Non-synonymous “target-site” mutations in Ace1 can confer resistance to both carbamate and OP insecticides by reducing how well they bind to AChE. However, these mutations make AChE less effective in the absence of insecticides. Due to the important role that AChE plays in the nervous system, these resistant phenotypes come with a high fitness cost.
Amplifications of the Ace1 gene have also been found to be associated with carbamate and OP resistance. It is thought that these amplifications, detectable using CNV analyses, may increase resistance phenotype by enabling more copies of the mutant gene to be present while restoring mosquito fitness in the absence of insecticides by allowing a un-mutated (wild-type) copy of the gene to remain.
Pirimiphos methyl (PM) is an OP insecticide used for indoor residual spraying (IRS). With the increase of pyrethroid and carbamate resistance, WHO has recommend PM for IRS since 2013. PM is the active ingredient in the IRS product Actellic CS, and is now being widely used, hence the importance of tracking known OP resistance markers.
Ace1 amino acid substitution frequencies#
Xavier Grau-Bové and colleagues demonstrated that, in Anopheles gambiae and colluzii from West Africa, the G280S amino acid altering SNP in the Ace1 gene was associated with resistance to the OP pirimiphos methyl (Grau-Bové et al. 2021).
Let’s examine Ace1 amino acid allele frequencies in An. coluzzi (West & Central Africa) from the Ag3.0 data release using the ag3.aa_allele_frequencies() function.
In brief, this analysis will take each amino acid in the Ace1 gene in turn, and calculate the frequency of alternative (non-reference) residues in each of our chosen mosquito cohorts.
For example, if we take the figure below and consider the locus of interest to be an amino acid in the Ace1 gene, we see that from our cohort of five individuals (ten haplotypes), there are four instances of an alternative amino acid. This means the allele frequency of the alternative amino acid here is 0.4.

A deep dive into the aa_allele_frequencies() function and allele frequencies in general can be found in workshop 1 - module 4, where we used it to investigate SNP and amino acid substitution frequencies in the Vgsc gene (the target of pyrethroid and DDT insecticides).
Using ? we can remind ourselves what parameters we need for this function.
ag3.aa_allele_frequencies?
Signature:
ag3.aa_allele_frequencies(
transcript: str,
cohorts: Union[str, Mapping[str, str]],
sample_query: Optional[str] = None,
sample_query_options: Optional[dict] = None,
min_cohort_size: Optional[int] = 10,
site_mask: Optional[str] = None,
sample_sets: Union[str, Sequence[str], NoneType] = None,
drop_invariant: bool = True,
include_counts: bool = False,
chunks: Union[int, str, Tuple[Union[int, str], ...], Callable[[Tuple[int, ...]], Union[int, str, Tuple[Union[int, str], ...]]]] = 'native',
inline_array: bool = True,
) -> pandas.core.frame.DataFrame
Docstring:
Compute amino acid substitution frequencies for a gene transcript.
Parameters
----------
transcript : str
Gene transcript identifier.
cohorts : str or Mapping[str, str]
Either a string giving the name of a predefined cohort set (e.g.,
"admin1_month") or a dict mapping custom cohort labels to sample
queries.
sample_query : str or None, optional
A pandas query string to be evaluated against the sample metadata, to
select samples to be included in the returned data.
sample_query_options : dict or None, optional
A dictionary of arguments that will be passed through to pandas
query() or eval(), e.g. parser, engine, local_dict, global_dict,
resolvers.
min_cohort_size : int or None, optional, default: 10
Minimum cohort size. Raise an error if the number of samples is less
than this value.
site_mask : str or None, optional
Which site filters mask to apply. See the `site_mask_ids` property for
available values.
sample_sets : sequence of str or str or None, optional
List of sample sets and/or releases. Can also be a single sample set
or release.
drop_invariant : bool, optional, default: True
If True, drop variants not observed in the selected samples.
include_counts : bool, optional, default: False
Include columns with allele counts and number of non-missing allele
calls (nobs).
chunks : int or str or tuple of int or str or Callable[[typing.Tuple[int, ...]], int or str or tuple of int or str], optional, default: 'native'
Define how input data being read from zarr should be divided into
chunks for a dask computation. If 'native', use underlying zarr
chunks. If a string specifying a target memory size, e.g., '300 MiB',
resize chunks in arrays with more than one dimension to match this
size. If 'auto', let dask decide chunk size. If 'ndauto', let dask
decide chunk size but only for arrays with more than one dimension. If
'ndauto0', as 'ndauto' but only vary the first chunk dimension. If
'ndauto1', as 'ndauto' but only vary the second chunk dimension. If
'ndauto01', as 'ndauto' but only vary the first and second chunk
dimensions. Also, can be a tuple of integers, or a callable which
accepts the native chunks as a single argument and returns a valid
dask chunks value.
inline_array : bool, optional, default: True
Passed through to dask `from_array()`.
Returns
-------
DataFrame
A dataframe of amino acid allele frequencies, one row per variant.
The variants are indexed by their amino acid change, their contig,
their position. The columns are split into two categories: there is
1 column for each cohort containing the frequency of the amino acid
change within the cohort, additionally there is a column `max_af`
containing the maximum frequency of the amino acide change accross
all cohorts; finally, there are 9 columns describing the variant
allele: `transcript` contains the gene transcript used for this
analysis, `effect` is the effect of the allele change, `impact`is
the impact of the allele change, `ref_allele` is the reference
allel, `alt_allele` is the alternate allele, `aa_pos` is the
position of the amino acid, `ref_aa` is the reference amino acid,
`alt_aa` is the altered amino acid with the varaint allele, and
`label` is the label of the variant allele.
Notes
-----
Cohorts with fewer samples than `min_cohort_size` will be excluded
from output data frame.
File: /home/conda/developer/35674e27b19f7c625ba32a1b88449ff45c90b40edb90a065b66c5a9a5388f41c-20250421-195247-360829-93-training-nb-maintenance-mgen-15.2.0/lib/python3.11/site-packages/malariagen_data/anoph/snp_frq.py
Type: method
ace1_aa_freqs_df = ag3.aa_allele_frequencies(
transcript="AGAP001356-RA",
sample_sets = '3.0',
cohorts="admin1_year",
sample_query='taxon == "coluzzii"'
)
ace1_aa_freqs_df
| frq_AO-LUA_colu_2009 | frq_BF-09_colu_2012 | frq_BF-09_colu_2014 | frq_CF-BGF_colu_1994 | frq_CI-LG_colu_2012 | frq_CM-CE_colu_2013 | frq_GH-AA_colu_2012 | frq_GH-CP_colu_2012 | frq_GH-WP_colu_2012 | frq_GM-L_colu_2006 | ... | transcript | aa_pos | ref_allele | ref_aa | alt_aa | effect | impact | alt_allele | max_af | label | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aa_change | contig | position | |||||||||||||||||||||
| R4G | 2R | 3489222 | 0.000000 | 0.006098 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 4.0 | C | R | G | NON_SYNONYMOUS_CODING | MODERATE | G | 0.006098 | R4G (2R:3,489,222 C>G) |
| P19T | 2R | 3489267 | 0.000000 | 0.000000 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 19.0 | C | P | T | NON_SYNONYMOUS_CODING | MODERATE | A | 0.018519 | P19T (2R:3,489,267 C>A) |
| G24C | 2R | 3489282 | 0.006173 | 0.000000 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 24.0 | G | G | C | NON_SYNONYMOUS_CODING | MODERATE | T | 0.006173 | G24C (2R:3,489,282 G>T) |
| G24S | 2R | 3489282 | 0.000000 | 0.000000 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 24.0 | G | G | S | NON_SYNONYMOUS_CODING | MODERATE | A | 0.018519 | G24S (2R:3,489,282 G>A) |
| V25L | 2R | 3489285 | 0.000000 | 0.000000 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 25.0 | G | V | L | NON_SYNONYMOUS_CODING | MODERATE | T | 0.020000 | V25L (2R:3,489,285 G>T) |
| ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
| V726M | 2R | 3493750 | 0.000000 | 0.000000 | 0.009434 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 726.0 | G | V | M | NON_SYNONYMOUS_CODING | MODERATE | A | 0.009434 | V726M (2R:3,493,750 G>A) |
| A731T | 2R | 3493765 | 0.000000 | 0.000000 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 731.0 | G | A | T | NON_SYNONYMOUS_CODING | MODERATE | A | 0.002907 | A731T (2R:3,493,765 G>A) |
| A731V | 2R | 3493766 | 0.000000 | 0.012195 | 0.009434 | 0.0 | 0.0125 | 0.0 | 0.000000 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 731.0 | C | A | V | NON_SYNONYMOUS_CODING | MODERATE | T | 0.012500 | A731V (2R:3,493,766 C>T) |
| V733A | 2R | 3493772 | 0.000000 | 0.006098 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.035714 | 0.00 | 0.0 | 0.0 | ... | AGAP001356-RA | 733.0 | T | V | A | NON_SYNONYMOUS_CODING | MODERATE | C | 0.035714 | V733A (2R:3,493,772 T>C) |
| R734T | 2R | 3493775 | 0.000000 | 0.000000 | 0.000000 | 0.0 | 0.0000 | 0.0 | 0.000000 | 0.02 | 0.0 | 0.0 | ... | AGAP001356-RA | 734.0 | G | R | T | NON_SYNONYMOUS_CODING | MODERATE | C | 0.020000 | R734T (2R:3,493,775 G>C) |
159 rows × 26 columns
We get 159 amino acid changes from our coluzzii cohorts, but many are at low frequencies. Let’s filter our DataFrame using query to just alternative amino acid frequencies greater than, or equal to 5%.
ace1_aa_filt_df = ace1_aa_freqs_df.query("max_af >= 0.05")
ace1_aa_filt_df
| frq_AO-LUA_colu_2009 | frq_BF-09_colu_2012 | frq_BF-09_colu_2014 | frq_CF-BGF_colu_1994 | frq_CI-LG_colu_2012 | frq_CM-CE_colu_2013 | frq_GH-AA_colu_2012 | frq_GH-CP_colu_2012 | frq_GH-WP_colu_2012 | frq_GM-L_colu_2006 | ... | transcript | aa_pos | ref_allele | ref_aa | alt_aa | effect | impact | alt_allele | max_af | label | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aa_change | contig | position | |||||||||||||||||||||
| F35S | 2R | 3489316 | 1.000000 | 0.975610 | 1.000000 | 1.000000 | 0.99375 | 1.000000 | 1.000000 | 1.00 | 1.000000 | 0.973684 | ... | AGAP001356-RA | 35.0 | T | F | S | NON_SYNONYMOUS_CODING | MODERATE | C | 1.000000 | F35S (2R:3,489,316 T>C) |
| G61D | 2R | 3489394 | 0.061728 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.041667 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | AGAP001356-RA | 61.0 | G | G | D | NON_SYNONYMOUS_CODING | MODERATE | A | 0.061728 | G61D (2R:3,489,394 G>A) |
| A65S | 2R | 3489405 | 1.000000 | 0.597561 | 0.660377 | 0.923077 | 0.70000 | 0.791667 | 0.678571 | 0.66 | 0.729167 | 0.315789 | ... | AGAP001356-RA | 65.0 | G | A | S | NON_SYNONYMOUS_CODING | MODERATE | T | 1.000000 | A65S (2R:3,489,405 G>T) |
| R106H | 2R | 3489529 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.208333 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | AGAP001356-RA | 106.0 | G | R | H | NON_SYNONYMOUS_CODING | MODERATE | A | 0.208333 | R106H (2R:3,489,529 G>A) |
| V218L | 2R | 3491888 | 0.000000 | 0.018293 | 0.028302 | 0.000000 | 0.00000 | 0.000000 | 0.035714 | 0.02 | 0.000000 | 0.000000 | ... | AGAP001356-RA | 218.0 | G | V | L | NON_SYNONYMOUS_CODING | MODERATE | T | 0.060000 | V218L (2R:3,491,888 G>T) |
| G280S | 2R | 3492074 | 0.000000 | 0.024390 | 0.018868 | 0.000000 | 0.43750 | 0.000000 | 0.285714 | 0.00 | 0.000000 | 0.000000 | ... | AGAP001356-RA | 280.0 | G | G | S | NON_SYNONYMOUS_CODING | MODERATE | A | 0.437500 | G280S (2R:3,492,074 G>A) |
| S338R | 2R | 3492329 | 0.000000 | 0.024390 | 0.000000 | 0.000000 | 0.00000 | 0.000000 | 0.000000 | 0.00 | 0.020833 | 0.000000 | ... | AGAP001356-RA | 338.0 | C | S | R | NON_SYNONYMOUS_CODING | MODERATE | A | 0.055556 | S338R (2R:3,492,329 C>A) |
| S367A | 2R | 3492414 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.000000 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | AGAP001356-RA | 367.0 | T | S | A | NON_SYNONYMOUS_CODING | MODERATE | G | 0.050000 | S367A (2R:3,492,414 T>G) |
| D624E | 2R | 3493273 | 0.000000 | 0.000000 | 0.000000 | 0.115385 | 0.01250 | 0.000000 | 0.035714 | 0.00 | 0.020833 | 0.000000 | ... | AGAP001356-RA | 624.0 | C | D | E | NON_SYNONYMOUS_CODING | MODERATE | A | 0.115385 | D624E (2R:3,493,273 C>A) |
| N645I | 2R | 3493401 | 0.000000 | 0.006098 | 0.009434 | 0.000000 | 0.01250 | 0.000000 | 0.035714 | 0.00 | 0.000000 | 0.000000 | ... | AGAP001356-RA | 645.0 | A | N | I | NON_SYNONYMOUS_CODING | MODERATE | T | 0.090909 | N645I (2R:3,493,401 A>T) |
| S648N | 2R | 3493410 | 0.000000 | 0.012195 | 0.009434 | 0.000000 | 0.00000 | 0.208333 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | AGAP001356-RA | 648.0 | G | S | N | NON_SYNONYMOUS_CODING | MODERATE | A | 0.208333 | S648N (2R:3,493,410 G>A) |
11 rows × 26 columns
Now we have just 11 amino acid substitutions of interest, including the G280S mutation implicated in organophosphate insecticide resistance.
Let’s display the pandas DataFrame as a heatmap for ease of visualisation.
ag3.plot_frequencies_heatmap(ace1_aa_filt_df, height=600, width=1000)
There are few high frequency amino acid substitutions in Ace1, as we might expect for a functionally constrained gene. However, we can see appreciable frequencies of the known organophosphate resistance marker G280S in cohorts from Cote d’Ivoire (CI-LG_colu_2012) and Ghana (GH-AA_colu_2012). There are also high frequencies of F35S and A65S mutations across all the cohorts. In the case of F35S, this may well be an artifact, driven by the mutant allele actually being in the reference genome so the wild-type (S) appears as the high frequency alternative allele. The A65F mutation is present in all populations at varying frequencies, but we can see that it is found at high frequency in the Central African Republic cohort collected in 1994, prior to OP use in malaria control.
Ace1 copy number variation frequencies#
Increases in copy number of the Ace1 gene have been linked with insecticide resistance in association with the G280S amino acid subsitution in both Anopheles and Culex mosquitoes.
In Culex, the 280S mutation has been shown to cause over 60% reduction in esterase enzymatic activity in the absence of insecticide, causing developmental and behavioural issues. It is likely that the same mutation causes similar issues in Anopheles.
As we can see in the figure below, a permanent “heterozygote” can be created by pairing a wild type 280G carrying Ace1 gene with at least one OP resistance mutation 280S carrying Ace1 gene, on the same haplotype.

This haplotypic heterozygote compensates for the neurophysiological fitness cost of 280S in the absence of insecticide. Plus there can be a number of copies of the resistant gene and carrying multiple copies has been strongly associated with increased carbamate resistance in bioassays (Edi et al. 2012).
Let’s use the CNV data to look into copy number of the the Ace1 gene in An. coluzzii from the Ag3.0 data set. We can use the ag3.plot_cnv_hmm_heatmap() function (explored in detail in workshop 2 - module 3 to visualise copy number around Ace1.
Briefly, this function builds a heatmap of genomic copy number per individual mosquito across a region of interest.
ag3.plot_cnv_hmm_heatmap?
Signature:
ag3.plot_cnv_hmm_heatmap(
region: Union[str, malariagen_data.util.Region, Mapping],
sample_sets: Union[str, Sequence[str], NoneType] = None,
sample_query: Optional[str] = None,
sample_query_options: Optional[dict] = None,
max_coverage_variance: Optional[float] = 0.2,
sizing_mode: Literal['fixed', 'stretch_width', 'stretch_height', 'stretch_both', 'scale_width', 'scale_height', 'scale_both'] = 'stretch_width',
width: Optional[int] = None,
row_height: int = 7,
track_height: Optional[int] = None,
palette: Optional[Sequence[str]] = ['#cccccc', '#5e3c99', '#b2abd2', '#f7f7f7', '#fdb863', '#e66101'],
genes_height: int = 90,
show: bool = True,
gene_labels: Optional[Mapping[str, str]] = None,
gene_labelset: Optional[bokeh.models.annotations.labels.LabelSet] = None,
) -> Optional[bokeh.model.model.Model]
Docstring:
Plot CNV HMM data for multiple samples as a heatmap, with a genes track,
using bokeh.
Parameters
----------
region : str or Region or Mapping
Region of the reference genome. Can be a contig name, region string
(formatted like "{contig}:{start}-{end}"), or identifier of a genome
feature such as a gene or transcript.
sample_sets : sequence of str or str or None, optional
List of sample sets and/or releases. Can also be a single sample set
or release.
sample_query : str or None, optional
A pandas query string to be evaluated against the sample metadata, to
select samples to be included in the returned data.
sample_query_options : dict or None, optional
A dictionary of arguments that will be passed through to pandas
query() or eval(), e.g. parser, engine, local_dict, global_dict,
resolvers.
max_coverage_variance : float or None, optional, default: 0.2
Remove samples if coverage variance exceeds this value.
sizing_mode : {'fixed', 'stretch_width', 'stretch_height', 'stretch_both', 'scale_width', 'scale_height', 'scale_both'}, optional, default: 'stretch_width'
Bokeh plot sizing mode, see also https://docs.bokeh.org/en/latest/docs
/user_guide/basic/layouts.html#sizing-modes.
width : int or None, optional
Plot width in pixels (px).
row_height : int, optional, default: 7
Plot height per row (sample) in pixels (px).
track_height : int or None, optional
Main track height in pixels (px).
palette : sequence of str or None, optional, default: ['#cccccc', '#5e3c99', '#b2abd2', '#f7f7f7', '#fdb863', '#e66101']
List of colors.
genes_height : int, optional, default: 90
Genes track height in pixels (px).
show : bool, optional, default: True
If true, show the plot. If False, do not show the plot, but return the
figure.
gene_labels : Mapping[str, str] or None, optional
A mapping of gene identifiers to custom labels, which will appear in
the plot.
gene_labelset : LabelSet or None, optional
A LabelSet to use in the plot.
Returns
-------
Model or None
Bokeh figure.
File: /home/conda/developer/35674e27b19f7c625ba32a1b88449ff45c90b40edb90a065b66c5a9a5388f41c-20250421-195247-360829-93-training-nb-maintenance-mgen-15.2.0/lib/python3.11/site-packages/malariagen_data/anoph/cnv_data.py
Type: method
ag3.plot_cnv_hmm_heatmap(
region='2R:3,300,000-4,000,000',
sample_query='taxon == "coluzzii"',
sample_sets='AG1000G-CI',
row_height=6
);
We can see that many of the coluzzii samples in the Cote d’Ivoire sample set have amplifications in the region of the Ace1 gene, of 3, or 4+ copies.
This fits with what we know from previous research showing that amplifications appear common in Cote d’Ivoire (Grau-Bové et al. 2021).
We can summarise the same data as frequency of amplification/deletion per cohort using the ag3.gene_cnv_frequencies() function that we learned about in workshop 2 - module 4.
Briefly, this function summarises gene amplification and deletion over each cohort of mosquitoes, so that we can estimate the fraction of mosquitoes from a given time, place and species that carry some form of copy number variation at a given gene of interest. It ignores whether the individual was heterozygous or homozygous for that CNV, and also ignores the actual number of additional copies that may be present.
ag3.gene_cnv_frequencies?
Signature:
ag3.gene_cnv_frequencies(
region: Union[str, malariagen_data.util.Region, Mapping, List[Union[str, malariagen_data.util.Region, Mapping]], Tuple[Union[str, malariagen_data.util.Region, Mapping], ...]],
cohorts: Union[str, Mapping[str, str]],
sample_query: Optional[str] = None,
sample_query_options: Optional[dict] = None,
min_cohort_size: int = 10,
max_coverage_variance: Optional[float] = 0.2,
sample_sets: Union[str, Sequence[str], NoneType] = None,
drop_invariant: bool = True,
include_counts: bool = False,
chunks: Union[int, str, Tuple[Union[int, str], ...], Callable[[Tuple[int, ...]], Union[int, str, Tuple[Union[int, str], ...]]]] = 'native',
inline_array: bool = True,
) -> pandas.core.frame.DataFrame
Docstring:
Compute modal copy number by gene, then compute the frequency of
amplifications and deletions in one or more cohorts, from HMM data.
Parameters
----------
region : str or Region or Mapping or list of str or Region or Mapping or tuple of str or Region or Mapping
Region of the reference genome. Can be a contig name, region string
(formatted like "{contig}:{start}-{end}"), or identifier of a genome
feature such as a gene or transcript. Can also be a sequence (e.g.,
list) of regions.
cohorts : str or Mapping[str, str]
Either a string giving the name of a predefined cohort set (e.g.,
"admin1_month") or a dict mapping custom cohort labels to sample
queries.
sample_query : str or None, optional
A pandas query string to be evaluated against the sample metadata, to
select samples to be included in the returned data.
sample_query_options : dict or None, optional
A dictionary of arguments that will be passed through to pandas
query() or eval(), e.g. parser, engine, local_dict, global_dict,
resolvers.
min_cohort_size : int, optional, default: 10
Minimum cohort size. Raise an error if the number of samples is less
than this value.
max_coverage_variance : float or None, optional, default: 0.2
Remove samples if coverage variance exceeds this value.
sample_sets : sequence of str or str or None, optional
List of sample sets and/or releases. Can also be a single sample set
or release.
drop_invariant : bool, optional, default: True
If True, drop variants not observed in the selected samples.
include_counts : bool, optional, default: False
Include columns with allele counts and number of non-missing allele
calls (nobs).
chunks : int or str or tuple of int or str or Callable[[typing.Tuple[int, ...]], int or str or tuple of int or str], optional, default: 'native'
Define how input data being read from zarr should be divided into
chunks for a dask computation. If 'native', use underlying zarr
chunks. If a string specifying a target memory size, e.g., '300 MiB',
resize chunks in arrays with more than one dimension to match this
size. If 'auto', let dask decide chunk size. If 'ndauto', let dask
decide chunk size but only for arrays with more than one dimension. If
'ndauto0', as 'ndauto' but only vary the first chunk dimension. If
'ndauto1', as 'ndauto' but only vary the second chunk dimension. If
'ndauto01', as 'ndauto' but only vary the first and second chunk
dimensions. Also, can be a tuple of integers, or a callable which
accepts the native chunks as a single argument and returns a valid
dask chunks value.
inline_array : bool, optional, default: True
Passed through to dask `from_array()`.
Returns
-------
DataFrame
A dataframe of CNV amplification (amp) and deletion (del)
frequencies in the specified cohorts, one row per gene and CNV type
(amp/del).
File: /home/conda/developer/35674e27b19f7c625ba32a1b88449ff45c90b40edb90a065b66c5a9a5388f41c-20250421-195247-360829-93-training-nb-maintenance-mgen-15.2.0/lib/python3.11/site-packages/malariagen_data/anoph/cnv_frq.py
Type: method
ace1_cnv_freqs_df = ag3.gene_cnv_frequencies(
region="2R:3,483,099-3,497,400",
sample_sets = '3.0',
cohorts="admin1_year",
sample_query='taxon == "coluzzii"'
)
ace1_cnv_freqs_df
| gene_strand | gene_description | contig | start | end | frq_AO-LUA_colu_2009 | frq_BF-09_colu_2012 | frq_BF-09_colu_2014 | frq_CF-BGF_colu_1994 | frq_CI-LG_colu_2012 | ... | frq_GH-WP_colu_2012 | frq_GM-M_colu_2012 | frq_GN-N_colu_2012 | frq_ML-2_colu_2004 | frq_ML-2_colu_2014 | frq_ML-3_colu_2012 | frq_ML-4_colu_2004 | max_af | windows | label | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gene_id | gene_name | cnv_type | |||||||||||||||||||||
| AGAP001356 | ACE1 | amp | + | Acetylcholinesterase [Source:UniProtKB/Swiss-P... | 2R | 3483099 | 3497400 | 0.0 | 0.0375 | 0.037736 | 0.0 | 0.87013 | ... | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.038462 | 0.0 | 0.87013 | 48 | AGAP001356 (ACE1) amp |
1 rows × 22 columns
We only have one row in this DataFrame as there is just one gene (Ace1) within the region parameter we provided and there are only amplification CNVs (“amp”) detected in our cohorts, no deletions. Consequently, we don’t need to apply any filtering here.
Let’s visualise this DataFrame as a heatmap.
ag3.plot_frequencies_heatmap(ace1_cnv_freqs_df, height=300, width=1000)
This gene frequencies summary makes the CNV data much easier to interpret. We can now clearly see that Amplifications of the Ace1 gene appear rare in our coluzzii samples, apart from a cohort from Ghana and the cohort from Cote d’Ivoire.
Combining SNP and CNV allele frequencies#
Given that an association between the G280S substitution, Ace1 gene amplification, and insecticide resistance has been shown, it will be useful to visualise the amino acid (SNP) frequencies and the CNV gene frequencies together.
You may have noticed that the frequency DataFrame output is similar for both ag3.aa_allele_frequencies(() and ag3.gene_cnv_frequencies(), with the cohort frequency columns named with a string starting with “frq”. This makes it easy to combine the outputs from our two analyses and visualise the results together.
First we simply combine the two DataFrames along the rows axis (axis=0) using pandas.concat().
ace1_combined_df = pd.concat(
[ace1_aa_filt_df, ace1_cnv_freqs_df],
axis=0, # concatenate rows
)
ace1_combined_df
| frq_AO-LUA_colu_2009 | frq_BF-09_colu_2012 | frq_BF-09_colu_2014 | frq_CF-BGF_colu_1994 | frq_CI-LG_colu_2012 | frq_CM-CE_colu_2013 | frq_GH-AA_colu_2012 | frq_GH-CP_colu_2012 | frq_GH-WP_colu_2012 | frq_GM-L_colu_2006 | ... | impact | alt_allele | max_af | label | gene_strand | gene_description | contig | start | end | windows | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F35S | 2R | 3489316 | 1.000000 | 0.975610 | 1.000000 | 1.000000 | 0.99375 | 1.000000 | 1.000000 | 1.00 | 1.000000 | 0.973684 | ... | MODERATE | C | 1.000000 | F35S (2R:3,489,316 T>C) | NaN | NaN | NaN | NaN | NaN | NaN |
| G61D | 2R | 3489394 | 0.061728 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.041667 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | MODERATE | A | 0.061728 | G61D (2R:3,489,394 G>A) | NaN | NaN | NaN | NaN | NaN | NaN |
| A65S | 2R | 3489405 | 1.000000 | 0.597561 | 0.660377 | 0.923077 | 0.70000 | 0.791667 | 0.678571 | 0.66 | 0.729167 | 0.315789 | ... | MODERATE | T | 1.000000 | A65S (2R:3,489,405 G>T) | NaN | NaN | NaN | NaN | NaN | NaN |
| R106H | 2R | 3489529 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.208333 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | MODERATE | A | 0.208333 | R106H (2R:3,489,529 G>A) | NaN | NaN | NaN | NaN | NaN | NaN |
| V218L | 2R | 3491888 | 0.000000 | 0.018293 | 0.028302 | 0.000000 | 0.00000 | 0.000000 | 0.035714 | 0.02 | 0.000000 | 0.000000 | ... | MODERATE | T | 0.060000 | V218L (2R:3,491,888 G>T) | NaN | NaN | NaN | NaN | NaN | NaN |
| G280S | 2R | 3492074 | 0.000000 | 0.024390 | 0.018868 | 0.000000 | 0.43750 | 0.000000 | 0.285714 | 0.00 | 0.000000 | 0.000000 | ... | MODERATE | A | 0.437500 | G280S (2R:3,492,074 G>A) | NaN | NaN | NaN | NaN | NaN | NaN |
| S338R | 2R | 3492329 | 0.000000 | 0.024390 | 0.000000 | 0.000000 | 0.00000 | 0.000000 | 0.000000 | 0.00 | 0.020833 | 0.000000 | ... | MODERATE | A | 0.055556 | S338R (2R:3,492,329 C>A) | NaN | NaN | NaN | NaN | NaN | NaN |
| S367A | 2R | 3492414 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.000000 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | MODERATE | G | 0.050000 | S367A (2R:3,492,414 T>G) | NaN | NaN | NaN | NaN | NaN | NaN |
| D624E | 2R | 3493273 | 0.000000 | 0.000000 | 0.000000 | 0.115385 | 0.01250 | 0.000000 | 0.035714 | 0.00 | 0.020833 | 0.000000 | ... | MODERATE | A | 0.115385 | D624E (2R:3,493,273 C>A) | NaN | NaN | NaN | NaN | NaN | NaN |
| N645I | 2R | 3493401 | 0.000000 | 0.006098 | 0.009434 | 0.000000 | 0.01250 | 0.000000 | 0.035714 | 0.00 | 0.000000 | 0.000000 | ... | MODERATE | T | 0.090909 | N645I (2R:3,493,401 A>T) | NaN | NaN | NaN | NaN | NaN | NaN |
| S648N | 2R | 3493410 | 0.000000 | 0.012195 | 0.009434 | 0.000000 | 0.00000 | 0.208333 | 0.000000 | 0.00 | 0.000000 | 0.000000 | ... | MODERATE | A | 0.208333 | S648N (2R:3,493,410 G>A) | NaN | NaN | NaN | NaN | NaN | NaN |
| AGAP001356 | ACE1 | amp | 0.000000 | 0.037500 | 0.037736 | 0.000000 | 0.87013 | NaN | 0.428571 | 0.00 | 0.000000 | NaN | ... | NaN | NaN | 0.870130 | AGAP001356 (ACE1) amp | + | Acetylcholinesterase [Source:UniProtKB/Swiss-P... | 2R | 3483099.0 | 3497400.0 | 48.0 |
12 rows × 32 columns
We can visualise our concatenated DataFrame in a neat heatmap, but first we need to tidy up the columns.
The code below makes a list of all the frequency columns in our DataFrame, plus the “label” column.
frq_cols = [col for col in ace1_combined_df.columns if 'frq' in col]
frq_cols.append("label")
frq_cols
['frq_AO-LUA_colu_2009',
'frq_BF-09_colu_2012',
'frq_BF-09_colu_2014',
'frq_CF-BGF_colu_1994',
'frq_CI-LG_colu_2012',
'frq_CM-CE_colu_2013',
'frq_GH-AA_colu_2012',
'frq_GH-CP_colu_2012',
'frq_GH-WP_colu_2012',
'frq_GM-L_colu_2006',
'frq_GM-M_colu_2012',
'frq_GN-N_colu_2012',
'frq_ML-2_colu_2004',
'frq_ML-2_colu_2014',
'frq_ML-3_colu_2012',
'frq_ML-4_colu_2004',
'label']
Using square brackets and our frq_cols list, we can subset our DataFrame to just the columns we need.
ace1_combined_cols_df= ace1_combined_df[frq_cols].copy()
ace1_combined_cols_df
| frq_AO-LUA_colu_2009 | frq_BF-09_colu_2012 | frq_BF-09_colu_2014 | frq_CF-BGF_colu_1994 | frq_CI-LG_colu_2012 | frq_CM-CE_colu_2013 | frq_GH-AA_colu_2012 | frq_GH-CP_colu_2012 | frq_GH-WP_colu_2012 | frq_GM-L_colu_2006 | frq_GM-M_colu_2012 | frq_GN-N_colu_2012 | frq_ML-2_colu_2004 | frq_ML-2_colu_2014 | frq_ML-3_colu_2012 | frq_ML-4_colu_2004 | label | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F35S | 2R | 3489316 | 1.000000 | 0.975610 | 1.000000 | 1.000000 | 0.99375 | 1.000000 | 1.000000 | 1.00 | 1.000000 | 0.973684 | 0.976744 | 1.000000 | 1.000000 | 0.962963 | 1.000000 | 0.98 | F35S (2R:3,489,316 T>C) |
| G61D | 2R | 3489394 | 0.061728 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.041667 | 0.000000 | 0.00 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00 | G61D (2R:3,489,394 G>A) |
| A65S | 2R | 3489405 | 1.000000 | 0.597561 | 0.660377 | 0.923077 | 0.70000 | 0.791667 | 0.678571 | 0.66 | 0.729167 | 0.315789 | 0.363372 | 0.909091 | 0.500000 | 0.648148 | 0.537037 | 0.56 | A65S (2R:3,489,405 G>T) |
| R106H | 2R | 3489529 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.208333 | 0.000000 | 0.00 | 0.000000 | 0.000000 | 0.002907 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00 | R106H (2R:3,489,529 G>A) |
| V218L | 2R | 3491888 | 0.000000 | 0.018293 | 0.028302 | 0.000000 | 0.00000 | 0.000000 | 0.035714 | 0.02 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.06 | V218L (2R:3,491,888 G>T) |
| G280S | 2R | 3492074 | 0.000000 | 0.024390 | 0.018868 | 0.000000 | 0.43750 | 0.000000 | 0.285714 | 0.00 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.018519 | 0.00 | G280S (2R:3,492,074 G>A) |
| S338R | 2R | 3492329 | 0.000000 | 0.024390 | 0.000000 | 0.000000 | 0.00000 | 0.000000 | 0.000000 | 0.00 | 0.020833 | 0.000000 | 0.014535 | 0.000000 | 0.000000 | 0.018519 | 0.055556 | 0.02 | S338R (2R:3,492,329 C>A) |
| S367A | 2R | 3492414 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00000 | 0.000000 | 0.000000 | 0.00 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.050000 | 0.000000 | 0.000000 | 0.02 | S367A (2R:3,492,414 T>G) |
| D624E | 2R | 3493273 | 0.000000 | 0.000000 | 0.000000 | 0.115385 | 0.01250 | 0.000000 | 0.035714 | 0.00 | 0.020833 | 0.000000 | 0.005814 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.00 | D624E (2R:3,493,273 C>A) |
| N645I | 2R | 3493401 | 0.000000 | 0.006098 | 0.009434 | 0.000000 | 0.01250 | 0.000000 | 0.035714 | 0.00 | 0.000000 | 0.000000 | 0.005814 | 0.000000 | 0.090909 | 0.000000 | 0.000000 | 0.04 | N645I (2R:3,493,401 A>T) |
| S648N | 2R | 3493410 | 0.000000 | 0.012195 | 0.009434 | 0.000000 | 0.00000 | 0.208333 | 0.000000 | 0.00 | 0.000000 | 0.000000 | 0.002907 | 0.045455 | 0.000000 | 0.000000 | 0.000000 | 0.00 | S648N (2R:3,493,410 G>A) |
| AGAP001356 | ACE1 | amp | 0.000000 | 0.037500 | 0.037736 | 0.000000 | 0.87013 | NaN | 0.428571 | 0.00 | 0.000000 | NaN | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.038462 | 0.00 | AGAP001356 (ACE1) amp |
Now we can simply use the ag3.plot_frequencies_heatmap() function to visualise.
ag3.plot_frequencies_heatmap(ace1_combined_cols_df, height=700, width=1000)
With the amino acid (SNP) and CNV frequencies combined, we can clearly see a relationship between the G280S mutation and Ace1 gene amplification.
There is a missing value for the “CM-CE” cohort in the CNV row. Missing cohort frequency values happen occasionally with CNV data due to quality control filtering.
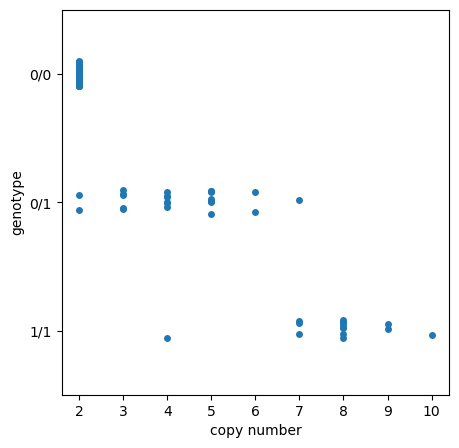
Calling SNPs at loci where there are CNVs is tricky, as the SNP calling model is expecting diploidy, but the CNV effectively makes an individual polyploid at that position. As we can see in the plot below, as copy number of Ace1 increases, it get’s less likely that the true heterozygote genotype is called.
Fortunately, the exact frequencies are not necessary, as being able to track the increase or decrease of these linked loci through time and space is what is important for informing insecticide use in vector control.
def genotype_by_copy_number(contig, position, sample_sets):
import seaborn as sns
import numpy as np
import matplotlib.pyplot as plt
geno = ag3.snp_genotypes(region=f"{contig}:{position}-{position+1}", sample_sets=sample_sets).compute()
geno = geno[0]
geno = np.apply_along_axis(arr=geno, func1d=lambda x: "/".join(map(str, x)), axis=1)
ds_cnv = ag3.gene_cnv(region=f"{contig}:{position}-{position+1}", sample_sets=sample_sets)
cnv_cn = ds_cnv['CN_mode'].compute().values[0]
fig, ax = plt.subplots(1,1, figsize=[5,5])
sns.stripplot(x=cnv_cn, y=geno, order = ['0/0','0/1','1/1'], ax=ax)
plt.ylabel("genotype")
plt.xlabel("copy number")
plt.show()
genotype_by_copy_number(contig="2R", position=3492074, sample_sets="AG1000G-GH")
What then now?#
Here we have demonstrated techniques that allow us to compare the frequency of SNPs and CNVs from the Ag3.0 data release together. However, the real power of these techniques to advance malaria control will come when they are applied to more recent data releases containing samples collected recently as spatial or temporal transects. With these data, associated SNPs and CNVs can be tracked together over space and time.
These analyses will need to be used in concert with others which are able to detect new loci under selection (see module 2 in this workshop). This is more relevant than ever with the large uptake in Actellic use (an organophosphate) for indoor residual spraying, as the established OP resistance mechanisms are likely to be supplemented by new loci and markers as mosquitoes evolve stronger resistance through greater exposure.
Practical exercise - Gste2#
English#
The Gste2 gene encodes an epsilon-class glutathione-S-transferase. It was initially studied in An. gambiae because its ortholog in the dengue and yellow fever vector Aedes aegypti has been linked to DDT resistance through increased gene expression.
One study looking at amino acid substitions in An. gambiae Gste2 found two mutations that in some cohorts were associated with insecticide resistance (Lucas et al. 2019). L119V was associated with permethrin (a type of pyrethroid) resistance and I114T was associated with both permethrin and DDT. Previously, Mitchell et al. (2014) found that Gste2 I114T conferred DDT resistance in An. gambiae and An. coluzzii.
The I114T mutation was also found to be associated with a CNV amplification that appeared to have fixed heterozygosity, much like with Ace1. The gene had duplicated, so that a wild-type gene and a I114T resistance mutation carrying gene were found on the same haplotype.
To further complicate the insecticide resistance story of Gste2 (and bring it into the scope of this module!) it has also been shown that overexpression of Gste2 is associated with OP resistance (Adolfi et al. 2019).
So, for this module’s practical excercise, I would like you run a case study on amino acid and CNV frequencies in Gste2, using the same analytical approach as we used for Ace1 and the An. coluzzii samples from Ag3.0.
1. Investigate amino acid frequencies in Gste2. HINT: transcript=”AGAP009194-RA”.
Why might the amino acid positional numbering go from high to low (opposite to Ace1)?
2. Examine CNVs in this region. HINT: region=”3R:28,597,652-28,598,640”
Plot a
cnv_hmm_heatmapfor the region - are there CNVs here?Investigate gene cnv frequencies for Gste2.
3. Concatenate the amino acid frequency and gene cnv frequency DataFrames.
4. Visualise the concatenated DataFrame as heatmap. HINT: don’t forget to subset the DataFrame columns first.
Are there any clear patterns of association between SNP and CNV markers of insecticide resistance, like we found for Ace1? Why might this be the case?
5. If you would like to dig deeper into these analyses and their interpretations…
As an optional exercise, the Ace1 and Gste2 case studies could be repeated but looking at An. gambiae instead of An. coluzzii. HINT: remember the range of An. gambiae is larger than coluzzii.
Français#
Gste2 code pour une glutathion-S-transférase de classe epsilon. Il a été initialement étudié chez An. gambiae car son orthologue chez le vecteur de la dengue et de la fièvre jaune Aedes aegypti, a été connecté à une résistance au DDT par augmentation de l’expression génique.
Une étude observant les substitutions d’acides aminés chez An. gambiae pour Gste2 a trouvé deux mutations qui pour certaines cohortes étaient associées à une résistance aux insecticide (Lucas et al. 2019). L119V était associée à une résistance à la perméthrine (un type de pyrethrinoïde) et I114T était associée à une résistance à la fois à la perméthrine et au DDT. Précédemment, Mitchell et al. 2014 a montré que Gste2 I114T confère une résistance au DDT chez les An. gambiae et les An. coluzzii.
La mutation I114T était aussi associée à une amplification des CNVs, qui semble avoir une hétérozygotie fixée, de manière très similaire à Ace1. Ce gène était dupliquée, de telle sorte qu’un gène contenant l’allèle sauvage et un gène contenant I114T, l’allèle de résistance se trouvaient dans le même haplotype.
Pour compliquer encore l’histoire de la résistance aux insecticides liée à Gste2 (et la rapprocher du sujet de ce module!), il a aussi été montré que la surexpression de Gste2 est associée à une résistance aux OPs. (Adolfi et al. 2019).
Donc, pour l’exercice pratique de ce module, je voudrais que vous étudiez les fréquences des substitutions d’acides aminés et ses CNVs pour Gste2 en utilisant la même approche analytique que nous avons utilisée pour Ace1 et les échantillons de coluzzii d’Ag3.0.`
1. Etudier les fréquences des acides aminés pour Gste2. Indice: transcript=”AGAP009194-RA”.
Pourquoi est-ce que la numérotation des positions des acides aminés set décroissante (contrairement au cas d’Ace1)?
2. Examiner les CNVs dans cette région. Indice: region=”3R:28,597,652-28,598,640”
Afficher le diagramme
cnv_hmm_heatmappour cette région - quels CNVs sont présents?Etudier les fréquences des CNVs pour Gste2.
3. Concaténer les DataFrames des fréquences des acides aminés et des fréquences des CNVs.
4. Visualiser le DataFrame obtenu par concaténation sous forme de heatmap. Indice: ne pas oublier de sélectionner les colonnes avant.
Y a-t-il des motifs clairs d’association enter les SNPs et les CNVs associés à la résistance aux insecticides, comme observé pour Ace1? Pourquoi cela?
5. Si vous souhaitez explorer ces analyses et leurs interprétations plus en profondeur…
En tant qu’exercice optionel, les études de cas pour Ace1 et Gste2 peuvent être répétées pour An. gambiae au lieu d’ An. coluzzii. Indice: se souvenir que la distribution des An. gambiae couvre une région plus vaste que celle des coluzzii.
Well done!#
In this module we have learnt how to:
Understand mechanisms and markers of organophosphate resistance.
Run amino acid frequency and CNV gene frequency analyses.
Combine and interpret SNP and CNV frequency data.
Exercises#
English#
Open this notebook in Google Colab and run it for yourself from top to bottom. As you run through the notebook, cell by cell, think about what each cell is doing, and try the practical exercises along the way.
Have go at the practical exercises, but please don’t worry if you don’t have time to do them all during the practical session, and please ask the teaching assistants for help if you are stuck.
Hint: To open the notebook in Google Colab, click the rocket icon at the top of the page, then select “Colab” from the drop-down menu.
Français#
Ouvrir ce notebook dans Google Colab et l’exécuter vous-même du début à la fin. Pendant que vous exécutez le notebook, cellule par cellule, pensez à ce que chaque cellule fait et essayez de faire les exercices quand vous les rencontrez.
Essayez de faire les exercices mais ne vous inquiétez pas si vous n’avez pas le temps de tout faire pendant la séance appliquée et n’hésitez pas à demander aux enseignants assistants si vous avez besoin d’aide parce que vous êtes bloqués.
Indice: Pour ouvrir le notebook dans Google Colab, cliquer sur l’icône de fusée au sommet de cette page puis choisissez “Colab” dans le menu déroulant.
